Lynda Spelman
Veracity Clinical Research, Australia
Title: National dermatology radiation oncology registry: A human research registry to collect information from patients referred to radiation oncologists for the management of non-melanoma and melanoma skin cancer and difficult to manage inflammatory skin diseases through radiotherapy
Biography
Biography: Lynda Spelman
Abstract
Rationale: Radiotherapy is the use of ionizing radiation to treat cancer and related diseases and has often been utilized as a treatment option for skin cancers. The registry will be used to analyse and assist in the development of best clinical practice for dermatologists and radiation oncologists.
Aim: To objectively measure baseline characteristics, e.g., demographic, medical history, treatment schedule, outcomes and follow-up of patients undergoing radiotherapy for skin cancers.
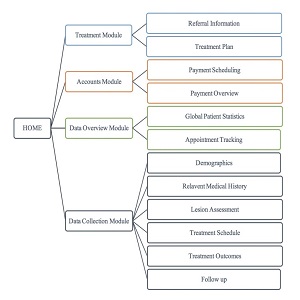
Registry Design: The registry design provides an accurate, non-interventionist approach to documenting patient outcomes and information. The Online Secure Platform (OSP) has four key modules (see Figure 1).
Study Schedule: Data collection will take place at the Registry enrolment by Referring Doctors (RD), treatment planning with Treating Radiation Oncologists (TRO), during treatment visit, and approximately every 3 months for the first 12 months and then 6 monthly thereafter.
Data Security & Handling: Data from the registry will be stored through the OSP on an Australian server accessible by an encrypted online portal, accessible only by the direct RD and TROs and their staff.
Monitoring Plan: A trained representative will monitor registry data entry, patient visit status, accuracy of information and missing data. All changed information, including the date and persons performing the corrections, will be available via the audit trail.
Multi-site Research Logistics: This study involves a large number of sites both by RDs and TROs. RDs are located in over 20 sites in Australia. TROs are located at Genesis Cancer Care sites across Australia.
Statistical Analysis: Registry reports will be written detailing the objectives, methodology, results, and conclusions. Bellberry human ethics will be consulted in this process.
Significance: The creation of a functional registry is not a straight forward process. A detailed, obsessional, establishment is necessary to ensure that the future use of its data is relevant